Pfizer MEVPRO Prostate Cancer Clinical Trials
Together we can make a difference
For information about the MEVPRO study medicine, please watch the short video below.
Prostate cancer is one of the most common types of cancer in men. About 1 in 8 men will be diagnosed with prostate cancer in their lifetime. About 1 in 44 men will die of prostate cancer.1
Doctors and scientists have significantly advanced treatments for men with prostate cancer over the last 10 years, but there is more work to be done. Pfizer is conducting a series of clinical trials to find out if a study medicine is safe and works to slow down or stop the growth of metastatic prostate cancer.
Pfizer’s legacy in cancer treatment spans over decades, with a strong foundation in research and development that has allowed us to study medicines in people since 1999.2 Pfizer’s prostate cancer pipeline seeks to further develop therapies to address the diverse needs of people living with prostate cancer.

MEVPRO is a series of clinical trials sponsored by Pfizer to evaluate the safety and effectiveness of a study medicine in treating metastatic prostate cancer. The investigational medicine has the potential to be the first-in-class EZH2 inhibitor for prostate cancer. It is taken as tablets by mouth twice-a-day.
For more information about EZH2, investigational medicine, and Pfizer’s MEVPRO Prostate Cancer Clinical Trials, please click the Learn More button below.
1 American Cancer Society: Facts & Figures 2024
2 TALZENNA® Prescribing Information. Pfizer Data on File.
Pfizer’s late-stage investigational medicine for metastatic prostate cancer
EZH2 Drives Prostate Cancers
Epigenetic dysregulation is a hallmark of cancer cells.
Enhancer of zeste homolog 2 (EZH2) is an epigenetic and histone methyltransferase regulator. People with prostate cancer with higher expression of the EZH2 gene are associated with a worse prognosis.
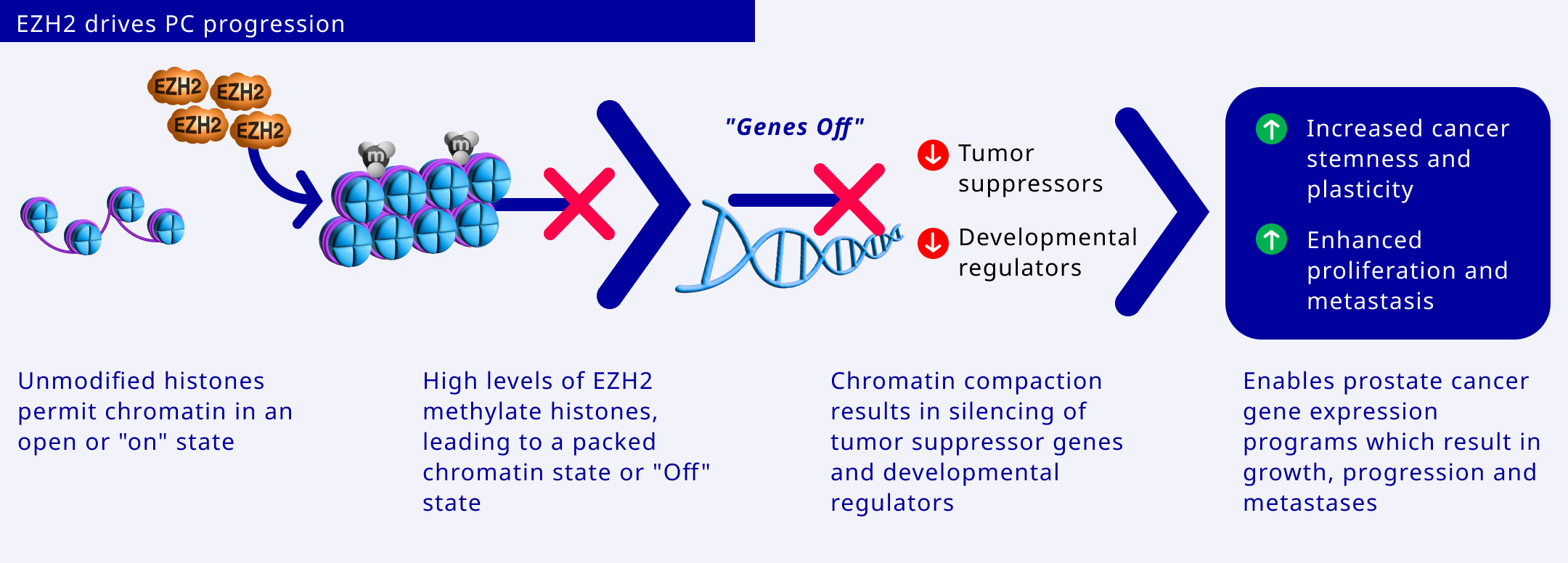
EZH2 drives prostate cancer growth and treatment resistance in a number of ways including:
- Silencing tumour suppressor genes
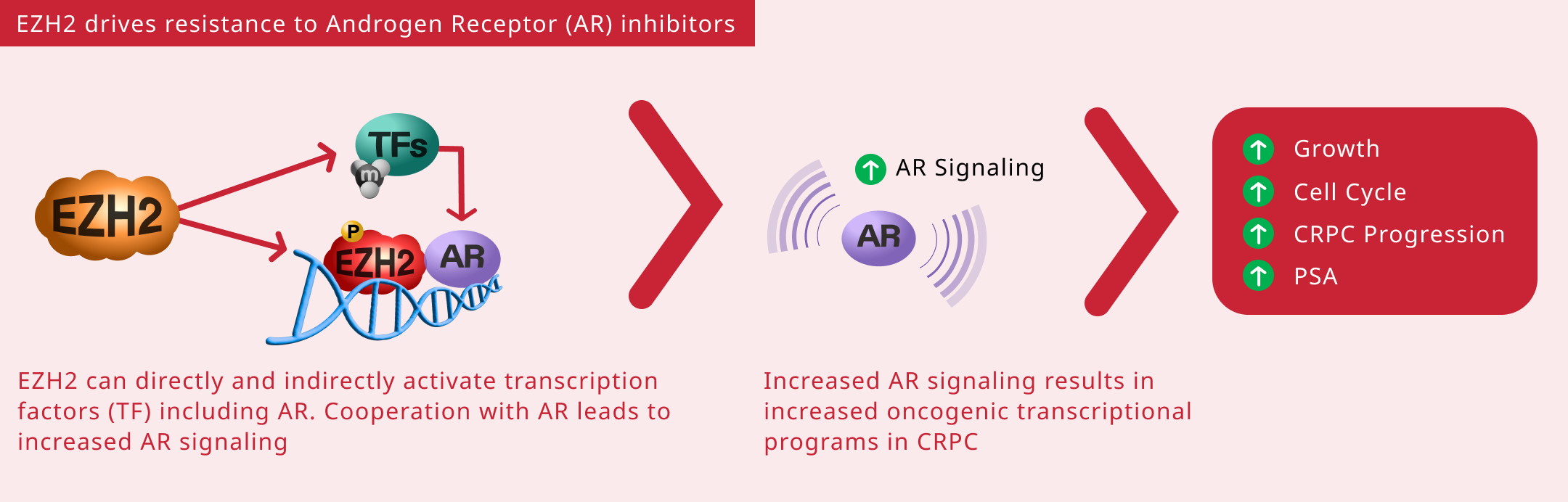
- Activating androgen receptor (AR) genes
- Methylating non-histone targets
EZH2 is considered to be a novel target for cancer treatment.
The investigational medicine is a selective, and orally bioavailable inhibitor of the protein EZH2.
In prostate cancer, it has demonstrated synergistic effects when combined with enzalutamide that may delay or prevent the emergence of enzalutamide-resistant NEPC state.
Investigational medicine + enzalutamide may restore gene functions repressed by both EZH2 and androgen receptors, resulting in downregulation of proliferation, survival, and cell cycle progression.
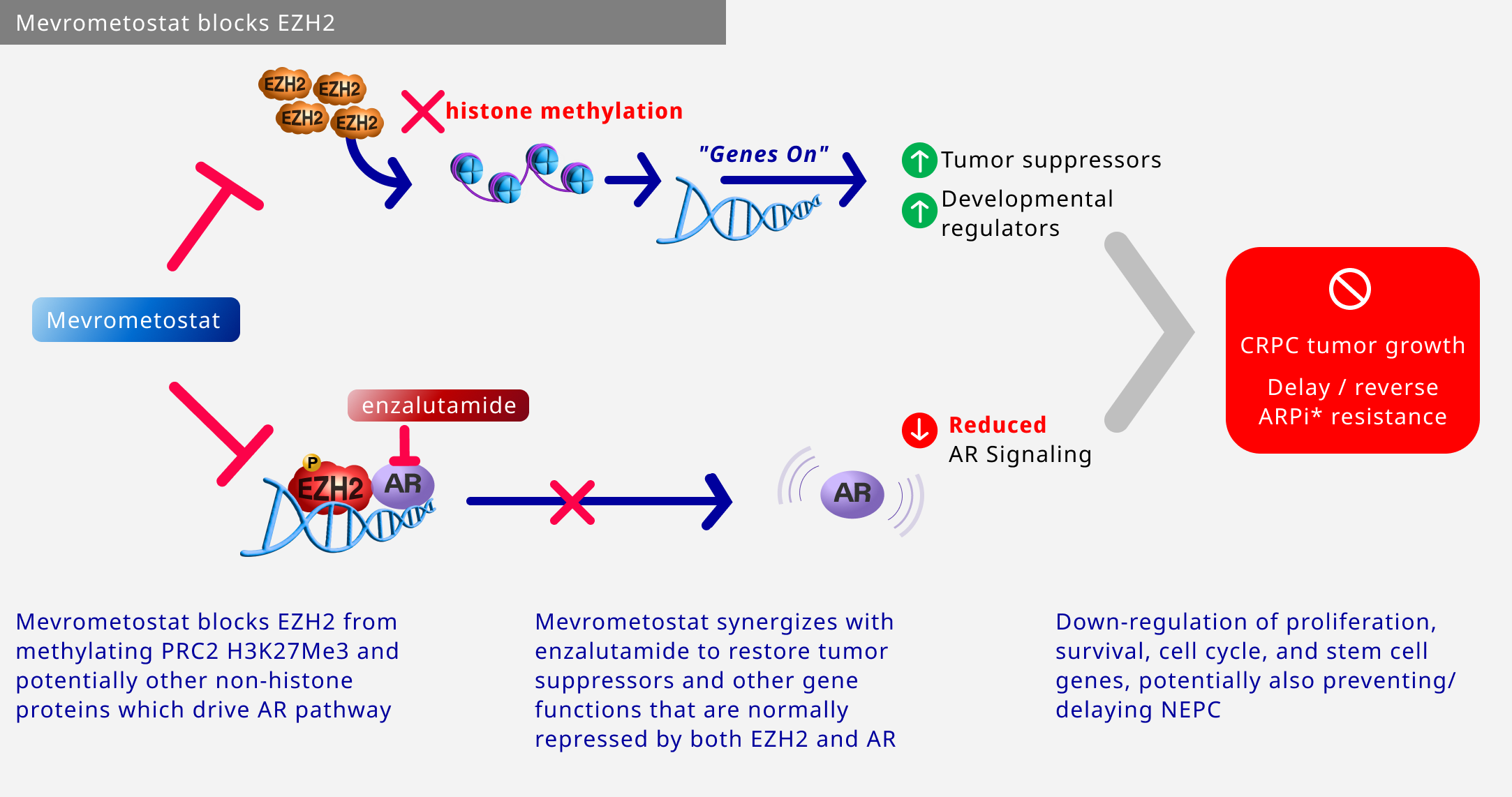
The study medicine is investigational and not approved by any health authority.
References
Bai Y, Zhang Z, Cheng L, et al. Inhibition of enhancer of zeste homolog 2 (EZH2) overcomes enzalutamide resistance in castration-resistant prostate cancer. J Biol Chem. 2019;294(25):9911-9923.
Du TQ, Liu R, Zhang Q, et al. EZH2 as a Prognostic Factor and Its Immune Implication with Molecular Characterization in Prostate Cancer: An Integrated Multi-Omics in Silico Analysis. Biomolecules. 2022;12(11):1617.
Gautam N, Kaur M, Kaur S. Structural assembly of Polycomb group protein and Insight of EZH2 in cancer progression: A review. J Cancer Res Ther. 2021;17(2):311-326.
Kaur P, Shankar E, Gupta S. EZH2-mediated development of therapeutic resistance in cancer. Cancer Lett. 2024;586:216706.
Kim J, Lee Y, Lu X, et al. Polycomb- and Methylation-Independent Roles of EZH2 as a Transcription Activator. Cell Rep. 2018;25(10):2808-2820.e4.
Kung PP, Bingham P, Brooun A, et al. Kung PP, Bingham P, Brooun A, et al. Optimization of orally bioavailable enhancer of zeste homolog 2 (Ezh2) inhibitors using ligand and property-based design strategies: identification of development candidate (R)-5,8-dichloro-7-(Methoxy(oxetan-3-yl)methyl)-2-((4-methoxy-6-methyl-2-oxo-1,2-dihydropyridin-3-yl)methyl)-3,4-dihydroisoquinolin-1(2h)-one(PF-06821497). J Med Chem. 2018;61(3):650-665.
Nouruzi S, Tabrizian N, Zoubeidi A. Beyond Expression: Role of Phosphorylated Residues of EZH2 in Lineage Plasticity in Prostate Cancer. Endocrinology. 2023;164(4):bqad023.
Park SH, Fong KW, Mong E, Martin MC, Schiltz GE, Yu J. Going beyond Polycomb: EZH2 functions in prostate cancer. Oncogene. 2021;40(39):5788-5798.
Ragavi R, Muthukumaran P, Nandagopal S, et al. Epigenetics regulation of prostate cancer: Biomarker and therapeutic potential. Urol Oncol. 2023;41(8):340-353.
Srinageshwar B, Maiti P, Dunbar GL, Rossignol J. Role of Epigenetics in Stem Cell Proliferation and Differentiation: Implications for Treating Neurodegenerative Diseases. Int J Mol Sci. 2016;17(2):199.
Xu K, Wu ZJ, Groner AC, et al. EZH2 oncogenic activity in castration-resistant prostate cancer cells is Polycomb-independent. Science. 2012;338(6113):1465-1469.
Zhao JC, Yu J, Runkle C, et al. Cooperation between Polycomb and androgen receptor during oncogenic transformation. Genome Res. 2012;22(2):322-331.
Do you have potentially eligible participants with metastatic prostate cancer in your care?

They may be eligible for one of Pfizer’s MEVPRO Prostate Cancer Clinical Trials.
To find out more about the MEVPRO clinical trials and how to refer potentially eligible participants, please use the site finder tool to find a site near you.
Find sites near you
We look forward to speaking with you and advancing prostate cancer research.
Why refer a person?
People are often introduced to the world of clinical trials through conversations with their healthcare provider. As with anything new, there may be a number of questions, concerns, or misconceptions. Being their trusted healthcare provider, we appreciate you taking the time to have an open discussion with people with prostate cancer and their loved ones who may be interested in contributing to research.
Clinical trials may be an option they will want to consider. While no benefit is guaranteed from an investigational medicine, people who participate in one of the MEVPRO clinical trials will contribute to the process of developing a potential new therapy.

If you would like more study-specific information to help inform conversations with potential participants, please let us know. Should any of them take part in the MEVPRO clinical trials, they will remain under your medical care for all non-study-related needs and will continue to receive any benefits to which they are entitled.
By referring potential participants who may be eligible to participate in one of Pfizer’s MEVPRO Prostate Cancer Clinical Trials, you are helping to further research on the treatment of metastatic prostate cancer.